
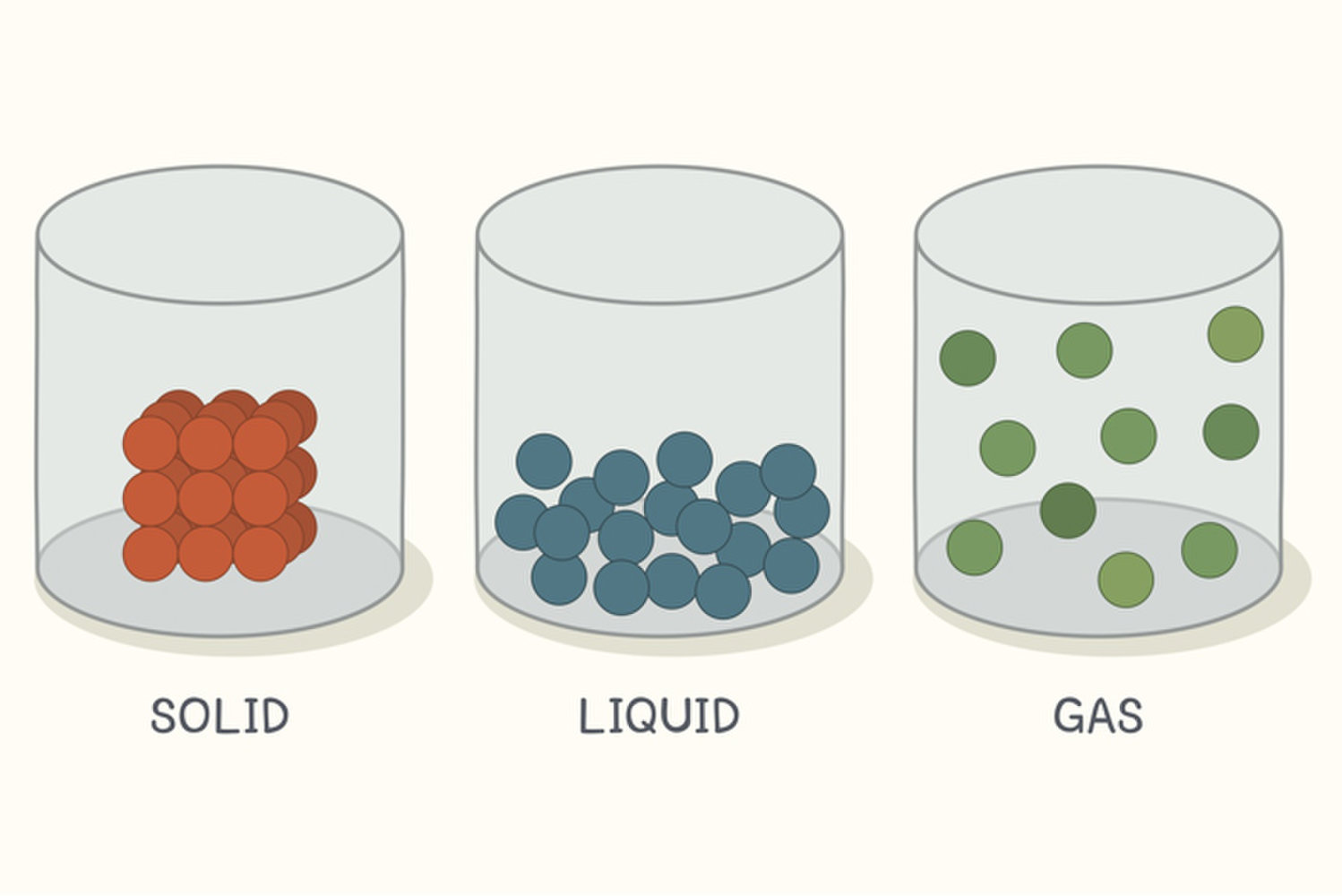
Due to the difference in types of unit cell every solid crystal is of different arrangement of particles. In a crystalline solid, the atoms, ions, or molecules are arranged in a definite repeating pattern, but occasional defects may occur in the pattern. Hint: Use the concept of crystal lattice and unit cell, where the unit cell which is the smallest portion if rotated in all three dimensions gives the big crystal lattice. Particles have no well-defined order structure (has random structure). How are the particles in a crystalline solid arranged.Formed when a saturated liquid is cooled slowly.A crystal posses long range order and symmetry. Particles (atoms, ions or molecules) are arranged in a regular repeating geometric arrangement. If the atoms or molecules are uniquely arranged in crystalline solid or liquid we call it as a crystal structure.(a) Deposition is a process in which a molecule changes directly from vapour to solid phase without passing through the liquid state. (a) Sublimation is a process in which a substance changes directly from solid to vapour phase without passing through the liquid state. The particles are held together too strongly to allow movement from place to place but the particles do vibrate about their position in the structure.
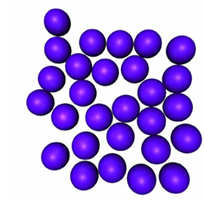
The particles in an amorphic solid are arranged in a somewhat random hap hazard manner. The particles (atoms, molecules or ions) in a crystalline solid are arranged in a regular repeating geometrical pattern in 3-Dimensional space.
The particles in a crystalline solid are arranged free#
When it reaches a temperature at which the particles have enough energy to overcome the interparticle attractive forces between themselves, particles are free to move.(a) Melting is a process in which a substance changes from solid to liquid phase. What is Crystalline A crystalline solid is that in which the constituent particles are orderly arranged in a three- dimensional pattern called the crystal. Incompressible because there is no empty space between the solid particles. Thus, the movement of the particles is restricted to vibration and rotation at its own axis. It is due to the strong interparticle attractive forces that hold the solid particles firmly in place.Solid particles are held by strong interparticle forces.

They are not able to move freely but can only vibrate and rotate about fixed position.Solid particles are closely packed together and in a rigid arrangement.


 0 kommentar(er)
0 kommentar(er)
